By Ariela Ruiz Caro, April 2021
A year and a month after that ill-fated March day when the World Health Organization (WHO) declared Covid-19 to be a pandemic, humanity now has available thirteen vaccines approved for emergency use. The pandemic, which has infected 133 million people and left 2.9 million dead, as well as inflicting unprecedented economic and social damage, sparked a race to develop treatments and/or vaccines to combat it. Never before had so many vaccines of such high efficacy been developed in so short a time to prevent a virus-caused disease.
Along with that success, however, 2020 brought a global economic fall of 3.4%, the deepest plunge after World War II. Latin America and the Caribbean suffered the harshest punishment, with negative growth reading 7.4%, leading to a 12% increase in poverty.
Still, as the vaccination process started up at the end of 2020, the new year brought hope for eradicating the pandemic. But that hope quickly gave way to the harsh reality of inequality and the resulting lack of access to the vaccine. Only five countries have had success with their vaccination programs: the United Arab Emirates (with 88% of the population vaccinated), Israel (61%), the United Kingdom (46.5%), Chile (36.7%) and the United States (32.1%).
Many nations, especially in Africa, have yet to start the vaccination process. And for most of those that have started, the process has been slow. That includes the European Union, even though the European Commission, representing 27 member countries, acquired its vaccine supply with a joint purchase, made well in advance to ensure better prices. The AstraZeneca, Pfizer/BioNTech and Johnson & Johnson laboratories, however, could not comply with the agreed-upon delivery dates, even as some of those countries found themselves threatened with a third wave of infections.
The situation in Latin America is even more serious. Only enough doses are available to vaccinate 5% of the population during a period of high infection rates and a rising death toll that have forced many countries in the region to reimpose confinement orders in the face of a collapse of its hospital services capabilities.
Global vaccine production is insufficient, and even that has been hoarded by developed countries that have bought up from two to three times more supply than is needed by their populations, an action that has come to be known as “vaccine nationalism.” What’s more, vaccine purchase does not necessarily equal vaccine availability, as has been the case in Europe and the cause of growing conflict there. These worrisome realities have not motivated developed countries to find a real solution to the need for increased supply. The proposals advanced to confront Covid-19, all of a voluntary character, have either encountered difficulties or failed outfight.
Proposals to Guarantee Equality in the Fight Against Covid-19
- The Covid-19 Vaccine Global Access Facility
On April 24, 2020, a new initiative was announced with the name of Access to Covid-19 Tools Accelerator(ACT-A) that calls for international cooperation to speed up the development and production of tests, treatments and vaccines against Covid-19 and to guarantee equitable access to them. The objective is for all nations, especially the poorest among them, to have the same access to testing for the coronavirus and to any treatment that may be discovered.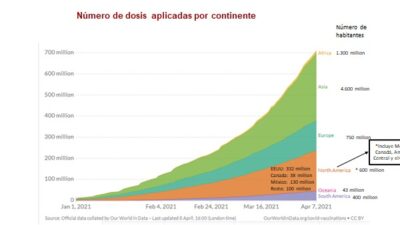
The most important pillar of this initiative is the Covid-19 Vaccine Global Access Facility (Covax), which seeks global financing with a view to equitable distribution. Co-led by the Vaccine Alliance (usually called Gavi for its previous name of Global Alliance for Vaccine and Immunization), with participation from the Bill and Melinda Gates Foundation, by the Coalition for Epidemic Preparedness Innovations (CEPI), and by the WHO, Covax was created to coordinate purchases at the international level in order to guarantee that the poorest countries are not left behind in the race to immunize 20% of the population in low- and medium-income nations. Its success would obviously depend on the delivery of vaccine doses and on financing to acquire them in the first place.
Currently, 190 countries make up Covax, of which some 90 have financed the development of a set of vaccines that once appeared to be quite promising. At first, the United States, China and Russia declined to participate in the initiative. The U.S. position under the Trump administration, as communicated by White House spokesperson Judd Deere in September 2020, was that “we will not be constrained by multilateral organizations influenced by the corrupt World Health Organization and China.”
On October 8, however, China chose to join the initiative at a time when it had three potential vaccines in Phase 3 clinical tests. The terms of its participation are not clear, but Hua Chunying, a spokesperson for China’s Foreign Relations Ministry, called it an important step toward assuring equitable distribution of vaccines, especially to developing nations.
Then, on January 21, one day after Joe Biden assumed the presidency, the U.S. government joined the mechanism. That about-face was followed on March 23 by a request from the Russian Direct Investment Fund to participate in Covax, with the understanding that it would give priority to supplying directly its own vaccine whose development it finances, Sputnik V, the most approved vaccine worldwide.
Despite good intentions, the mechanism has failed to live up to expectations. The problem is not in the financing but rather in the scarcity of the vaccines themselves, and their hoarding by rich nations, according to Mexican Foreign Relations Secretary Marcelo Ebrard. Speaking before the U.N. Security Council on February 17 for all Latin American and Caribbean nations, Ebrard urged developed nations to avoid hoarding vaccines and instead speed up their delivery to the Covax mechanism. “Covax has been insufficient so far,” he said. “The scenario we wanted to avoid has unfortunately been confirmed. To date, vaccines have not been distributed through this international mechanism.”
- A Covid-19 Repository of Rights
On May 29, 2020, WHO Director General Tedros Adhanom Ghebreyesus and Costa Rican President Carlos Alvarado together launched a project that would create a sort of open-access databank of all technology relevant to Covid-19. Known as C-TAP (for Covid-19 Technology Access Pool), the mechanism allows for the centralization and constant updating of the aggregate of knowledge available for the prevention, diagnosis and treatment of the disease.
The project implies a temporary and voluntary suspension of intellectual property rights for material related to Covid-19 prevention and treatment. It is based on the concept that “vaccines, tests, diagnoses, and other key tools in the response to the coronavirus should be universally available as global public goods.”
The two leaders issued a joint “Call to Solidarity” in which they asked the WHO member states, individual governments, researchers, research and development funds, and civil society organizations to unite behind the initiative. Ignoring that call was the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), which vehemently opposed the project even though many of them receive public funding or advance payments for their research from the governments of rich countries.
Just hours after Tedros and Alvarado called for international solidarity, U.S. President Donald Trump pulled his country out of the WHO, a move that was harshly criticized by the international community, especially by the U.S. allies in the European Union. In or out of the WHO, Trump opposed the C-TAP project, calling it a disincentive to innovation.
The rejection of the proposal and the difficulties looming over the Covax mechanism led Mustaqeem da Gama, South Africa’s representative before the World Trade Organization (WTO), to doubt whether poor countries can reach their full capacity while depending on the good will of the rich. “Philanthropy is fine up to a certain point,” he said, “but if we want to build capacity that goes beyond this particular pandemic . . . we need to invest resources locally, in technology transfer, in the ability to build.” Brajendra Navnit, India´s ambassador to the WTO, expressed in similar terms his belief that such initiatives as ACT-A and Covax are simply inadequate for the challenges we face.
- Temporary suspension of intellectual property rights at the WTO
A bolder scenario, proposed by India and South Africa in October 2020, to the WTO’s Council on the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS in English, or ADPIC in Spanish), called for temporarily suspending all intellectual property rights related to the treatment and prevention of Covid-19 until there is an arrangement that will deliver the vaccine to the entire world population. Even though the TRIPS already includes some foreseeable exceptions, such as the fact that for reasons of public interest, a nation can ask a patent holder to authorize a third party to produce a medication or vaccine, the process is complex and cumbersome. The initiative considers that the only way to satisfy global demand for vaccine doses is to facilitate knowledge acquisition by those countries that have the capacity to produce medications both for internal consumptions and exportation, without being sanctioned by the WTO for violating intellectual property norms. The proposal also prioritizes a fast-track process not only in order to prevent unnecessary deaths and more quarantines, but also to stop new mutations of the coronavirus.
This intellectual property rights suspension proposal was last debated on March 10 and 11. More than 100 of the 164 WTO member nations support it, as do at least 350 non-governmental organizations, among them Doctors Without Borders and Amnesty International, as well as individual members of the European Parliament and the U.S. Congress. Opposing it, however, are the home countries of the powerful pharmaceutical industry, such as the United States, Japan, the European Union, the United Kingdom, Switzerland and Canada. Augmenting vaccine production, as the initiative seeks to do, would lower prices and reduce the ability of the pharmaceutical labs to impose conditions on governments, as they do now. They sign agreements with confidentiality clauses that omit the prices of doses, the delivery schedules and the guarantees that the sellers demand and the governments are forced to accept, given their collapsing health systems and profound economic crises.
This proposal has the backing of the African Union, an intergovernmental policy and cooperation organization that brings together 56 countries on that continent. The union sent out a communication last February 22 supporting the temporary suspension promoted by India and South Africa before the WTO, calling it the most effective way to tackle the “artificial scarcity” resulting from “vaccine nationalism and market-driven mechanisms.” It also endorsed WHO Director General Tedros’s declaration that “allowing the majority of the world’s population to not be vaccinated not only perpetuates unnecessary illness and death, as well as well as prolonging existing quarantines, but will also generate new mutations of the virus as it continues to spread among unprotected populations.” As Dr. Tedros points out, those variants may not be able to be controlled by the vaccines available. Renewing his call for developed countries to support India and South Africa’s proposal, he said, “If not now, when?”
China and Russia’s Vaccine Diplomacy
No other Latin American country has come close to the advancements made by Chile’s vaccination program. As of April 5, 36% of that country’s population has received at least one dose and 20.1% have completed the vaccination regimen. About 90% of its vaccination program has been carried out with a vaccine from China’s Sinovac laboratory, with the clinical testing of the vaccine having taken place in Chile itself, in coordination with the Catholic University of Chile. Also, at the end of March, that country’s president unveiled an agreement reached to obtain 2 million doses from the Chinese lab CanSino Biologics, which only requires one dose to reach immunization.
Sinovac has also signed a vaccine technology transfer agreement with Brazil and is participating with the Butantan Institute of Sao Paulo in its manufacture. The supply of Chinese vaccines has led the Brazilian government to allow the Huawei company to participate in the bidding for the installation of 5G technology in Brazil, that had previously been vetoed because of pressure from the U.S. government.
As for the Russian vaccines, it became known in mid-March through a report by the Office of Governmental Affairs of the U.S. Department of Health and Human Services (HHS) that the Trump administration had in 2020 dissuaded Brazil from accepting the Russian Sputnik V vaccine and offered Panama technical assistance in exchange for refusing Cuban medications, those actions being intended to minimize threats from “ill-intentioned states.” Just days after the revelation, Brazil’s health ministry said it had gone ahead and signed an agreement to buy 10 million doses of the vaccination in question, though its authorization for emergency use is still being evaluated.
Argentina and Peru carried out clinical tests on vaccines from the Sinopharm laboratory, and both countries have received an initial delivery of a million doses, as part of an agreement for an eventual 30 million and 38 million doses, respectively. Argentina has also received Sputnik V vaccines manufactured by the Gamaleya lab, as well as Covishield vaccines from India’s Serum Institute, Oxford/AstraZeneca and CanSino Biologics. Peru’s vaccines have come from Pfizer and Sinopharm.
Mexico has claimed non-compliance by U.S. laboratories in the delivery of doses. Based on that problem, Mexican President Andrés Manuel López Obrador planned to ask Joe Biden, his U.S. counterpart, during a March 1 virtual meeting to share with Mexico part of the surplus vaccine supply stored in the United States. However, before the meeting even began, any possibility of the United States sharing its supply with its southern neighbor or any other country was ruled out, at least at this stage.
Days later, the Mexican government announced the purchase of 10 million doses of Sinovac and 12 million of Sinopharm. It has also signed an agreement with CanSino Biologics laboratory to package vaccines produced by that company, making Mexico the first country in the region to approve the CanSino Biologics vaccine, and the second overall, after China, to package it in the Drugmex plant in Querétaro. The Querétaro facility has the capacity to produce up to 85 million doses per year and during the current emergency will focus exclusively on packaging the vaccine in question, which requires only one dose. In should be noted as well that given the problematic conditions along the Mexico-U.S. border, the U.S. government has sent 2.5 million doses of the AstraZeneca vaccine to Mexico.
Save for Costa Rica, El Salvador, Panama and Cuba – none of which have approved any – all Latin American nations have approved the use of Russia’s Sputnik V vaccine, manufactured by the Gamaleya lab, or one of the four Chinese vaccines produced by CanSino, Sinopharm and Sinovac. The Cuban government chose to avoid falling into a war of vaccine purchases and instead decided to stake its fortunes on developing a home-grown product. It is the only Latin American country that has three vaccine projects going, two with the Finlay Vaccines Institute, and a third with the Centro de Ingeniería Genética y Biotecnología (CIGB) that is already in Phase 3 trials.
The Chinese and Russian vaccines are also being offered in Africa, Asia and even Europe, where some countries have distanced themselves from the joint purchasing strategy of the European Commission in favor of acquiring them on their own from those two nations.
This growing presence of China and Russia in the European vaccine market can be seen in the decision by some nations (Hungary, Serbia and Belarus) to opt for the vaccine produced by Sinopharm, or to approve the use of the Sputnik V vaccine (as is the case with Macedonia, Slovakia, the Czech Republic, Bosnia Herzegovina and Hungary). Recently, the Italian-Swiss pharmaceutical Adienne Pharma & Biotech the Russian Direct Investment Fund, which holds the patent, signed an agreement to produce the Sputnik V vaccine in Italy.
Unlike the United States, which has been vaccinating its own population at a dizzying pace, China and Russia, and India as well, have been working on selling its vaccines, as well as donating them, around the world. A side effect of that approach is a low vaccination rate among their own populations. China may be able to afford that luxury, since it has the pandemic under control locally, but Russia, with only 5% of its population vaccinated, hasn’t been able to reduce its Covid-19 death rate to under 400 a day. And the drastic increase of infections and deaths in India during March has led to delays in the delivery of its vaccines to the Covax mechanism.
As the WHO’s Dr. Tedros points out, “If a country could be named that has contributed more than any other on Earth in terms of sharing vaccines, it would be India.” But it’s understandable, he says, that at a time of raising cases, the Asian nation would need to use more vaccine doses locally. A similar situation can be seen with the supply of active pharmaceuticals to be sent from the Chinese Sinovac lab to Brazil’s Butantan Institute for production of the CoronaVac vaccine in Brazil. The Chinese government made the decision to vaccinate massively the Chinese population living near the border with India because of the rising case rates in the latter nation. That has of course temporarily reduced its capacity to send the pharmaceutical inputs to Brazil.
Brazil, for its part, with 4,000 Covid-19 deaths per day, has lost control over the pandemic, which its denialist president, Jair Bolsonaro, has long underestimated. As a result, Brazil represents a growing threat for the entire continent of South America. The WHO issued a statement concluding that the sudden spike in demand has created a scarcity of supplies such as glass vials, plastic filters, and essential raw materials that is limiting vaccine production and could pose a risk to the availability of routine non-Covid-related vaccinations of children. The WHO’s communiqué repeated its insistence that any solution to the pandemic must be global.
United Nations Secretary-General Antonio Gutierres, has used similar language in criticizing the excessive “stockpiling” of vaccine doses by developed countries. Gutierres has joined the chorus of global leaders calling for the sharing of the vaccines. His message is that humankind has the capacity to produce the weapons needed to defeat the pandemic if it will only act cooperatively with a common purpose.
However, the Western nations’ propensity to protect Big Pharma’s exorbitant profits impedes any cooperative global solution to this pandemic. Other vaccine-producing nations, such as China, Russia and India, consider their products to be “global public goods” and facilitate access to them as much as possible and use them to exercise their influence on the chessboard of geopolitics. That approach does not apply to the United States. Perhaps ironically, the strategic framework for the Western Hemisphere approved by the U.S. National Security Council under the Trump administration last August and aimed at limiting China and Russia’s influence in the region, has not been served by the “everyone in it for themselves” (as opposed to equitable) U.S. vaccine policy. On the contrary, it has sent Latin America into the arms of those supposedly “malignant” nations.
Ariela Ruiz Caro is an economist from Humboldt University of Berlin and holds a Master’s Degree in Economic Integration Processes from the University of Buenos Aires. She is an analyst of the Americas Program for the Andean/Southern Cone region.
Source https://www.counterpunch.org
